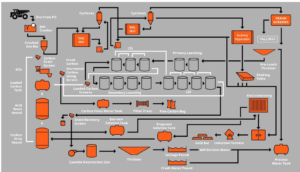
In the cyanidation gold extraction process, activated carbon is used to adsorb gold-cyanide complexes (Au(CN)₂⁻). However, the calcification of the activated carbon surface can significantly reduce its adsorption efficiency. Below is an analysis of the causes of activated carbon surface calcification and corresponding solutions.
—
### I. Analysis of Causes of Activated Carbon Surface Calcification
1. **Presence of Calcium Ions in the Solution**:
– In the cyanidation process, lime (Ca(OH)₂) or calcium hydroxide is commonly used to adjust the pulp pH to maintain cyanide stability in an alkaline environment (pH typically controlled at 10-11). This results in a high concentration of calcium ions (Ca²⁺) in the pulp.
– Calcium ions may react with other anions in the solution, such as carbonate (CO₃²⁻) or sulfate (SO₄²⁻), forming insoluble calcium salts (e.g., CaCO₃, CaSO₄) that deposit on the activated carbon surface.
2. **Formation of Calcium Carbonate**:
– Carbon dioxide (CO₂) from the air dissolves into the pulp, reacting with water to form carbonic acid (H₂CO₃), which dissociates into carbonate ions (CO₃²⁻). In a high-pH environment, carbonate ions combine with calcium ions to form calcium carbonate (CaCO₃) precipitate:
Ca²⁺ + CO₃²⁻ → CaCO₃↓
– Calcium carbonate deposits in the pores of activated carbon, clogging them and reducing adsorption capacity.
3. **Precipitation of Calcium Sulfate or Other Calcium Salts**:
– If the pulp contains sulfate ions (SO₄²⁻, possibly from the oxidation of sulfide minerals or added sulfates), calcium ions may form calcium sulfate (CaSO₄·2H₂O, gypsum) precipitate:
Ca²⁺ + SO₄²⁻ → CaSO₄↓
– These precipitates also accumulate on the activated carbon surface, contributing to calcification.
4. **Surface Properties of Activated Carbon**:
– Activated carbon has a high specific surface area and abundant pore structure, making it prone to adsorbing calcium ions or calcium salt particles. Once calcium salts nucleate and grow in the pores, they block the pore structure, hindering the adsorption of gold-cyanide complexes.
– Functional groups on the activated carbon surface may chemically or physically adsorb calcium ions, promoting calcium salt deposition.
5. **Process Operating Conditions**:
– **High Calcium Concentration**: Excessive lime addition leads to elevated calcium ion levels, increasing the likelihood of calcium salt precipitation.
– **Prolonged Operation**: Long-term use of activated carbon in the pulp causes gradual accumulation of calcium salts, exacerbating calcification.
– **Temperature and Mixing**: Higher temperatures or uneven mixing may accelerate calcium salt deposition.
6. **Synergistic Effects of Organic and Inorganic Substances**:
– The pulp may contain organic matter (e.g., flotation reagents, oils) or inorganic substances (e.g., silicates, sulfates), which can form complexes with calcium ions, further aggravating surface clogging of activated carbon.
—
II. Solutions to Activated Carbon Surface Calcification
To address the above causes, measures can be taken in process optimization, activated carbon treatment, and equipment improvement:
1. **Optimize Lime Dosage**:
– Precisely control lime addition to maintain the pulp pH in the optimal range (10-11), avoiding excessive calcium ion introduction.
– Use online pH monitoring and automated dosing systems for accurate control to minimize calcium ion concentration.
– Consider partially replacing lime with other alkaline agents (e.g., sodium hydroxide, NaOH) to reduce calcium ion input.
2. **Reduce Calcium Carbonate Formation**:
– **Control CO₂ Ingress**: Minimize air exposure in mixing or adsorption tanks, for example, by using sealed equipment or covers to reduce CO₂ dissolution.
– **Degassing Treatment**: Perform degassing of the pulp before it enters the adsorption system to remove dissolved CO₂, thereby reducing carbonate ion formation.
– **Add Inhibitors**: Introduce small amounts of chemicals that inhibit calcium salt crystallization (e.g., polyphosphates or organic phosphonates) to prevent nucleation and growth of calcium carbonate or sulfate.
3. **Improve Activated Carbon Selection and Pretreatment**:
– **Select Anti-Calcification Carbon**: Choose activated carbon with optimized pore structure and stable surface chemistry to reduce calcium ion adsorption and salt deposition.
– **Pretreat Activated Carbon**: Before use, wash activated carbon with dilute acid (e.g., HCl) or deionized water to remove potential calcium salts or impurities from the surface.
– **Modify Activated Carbon**: Chemically modify the carbon surface (e.g., by introducing hydrophobic groups) to reduce affinity for calcium ions and minimize salt deposition.
4. **Periodic Regeneration or Cleaning of Activated Carbon**:
– **Acid Washing**: Regularly clean activated carbon with dilute acid (e.g., 5%-10% hydrochloric acid or nitric acid) to dissolve surface-deposited calcium carbonate or sulfate. After acid washing, rinse with water to neutrality to avoid residual acid affecting subsequent adsorption:
CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂↑
– **Thermal Regeneration**: Regenerate activated carbon at high temperatures (600-800°C) to decompose calcium salts and restore pore structure. Thermal regeneration should be conducted in an inert atmosphere (e.g., nitrogen) to prevent carbon oxidation.
– **Ultrasonic Cleaning**: Use ultrasonic vibration to remove calcium salt deposits from the carbon surface, suitable for mild calcification.
5. **Optimize Adsorption Process Parameters**:
– **Shorten Carbon Usage Cycle**: Regularly replace or regenerate activated carbon to prevent severe calcification buildup.
– **Control Pulp Concentration**: Reduce the concentration of solids and organic matter in the pulp to minimize synergistic deposition with calcium ions.
– **Optimize Mixing Conditions**: Ensure uniform mixing in adsorption tanks to avoid localized high calcium ion concentrations or deposition.
6. **Improve Equipment Design**:
– **Multi-Stage Adsorption System**: Implement a multi-stage countercurrent adsorption process to reduce the calcification burden on individual carbon stages and improve overall adsorption efficiency.
– **Filtration Pretreatment**: Add filtration or sedimentation units before the pulp enters the adsorption system to remove some calcium salts and suspended solids.
– **Carbon Screening**: Periodically screen activated carbon to remove fine particles clogged by calcification, maintaining uniform particle size.
7. **Monitoring and Analysis**:
– Regularly monitor calcium ion, carbonate ion, and other relevant ion concentrations in the pulp, adjusting process parameters as needed.
– Periodically sample and analyze activated carbon (e.g., using SEM, XRD, or BET surface area testing) to assess calcification extent and optimize regeneration frequency.
—
### III. Precautions
1. **Safety**:
– Ensure operational safety during acid washing or chemical treatments to prevent cyanide leaks or acid-related hazards.
– Treat waste liquids in compliance with environmental regulations to avoid pollution from calcium salts or other chemicals.
2. **Balancing Cost and Effectiveness**:
– Methods like acid washing and thermal regeneration are effective but increase operational costs. Evaluate their necessity based on calcification severity and gold adsorption efficiency.
– Prioritize process optimization and preventive measures to minimize calcification occurrence.
3. **Variability in Practical Applications**:
– Ore properties (e.g., sulfide or organic content) vary across gold mines, affecting calcification severity. Solutions should be tailored to specific conditions.
– For high-calcium or carbonate-containing ores, calcification may be more pronounced, requiring enhanced pretreatment.
—
IV. Summary
The primary cause of activated carbon surface calcification is the formation of insoluble calcium salts (e.g., calcium carbonate or sulfate) from calcium ions reacting with carbonate, sulfate, or other ions, which deposit in the carbon pores. Solutions include optimizing lime dosage, reducing CO₂ ingress, improving carbon selection and regeneration, and fine-tuning process parameters. By implementing comprehensive measures, calcification can be effectively mitigated, enhancing activated carbon adsorption efficiency and the economic viability of the cyanidation gold extraction process.